Teething Gels for Infants – FDA Warning
Do not use teething gels in infants.
A warning per the Federal Drug Administration (FDA)
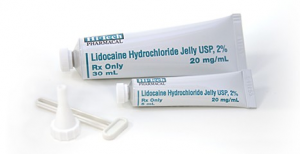 The FDA has recently issued a statement warning against the use of prescription numbing agents in teething children. Specifically the use of viscous lidocaine has been shown to potentially cause serious harm (including seizure, brain injury, and heart problems) when used on infants and young children as a treatment for discomfort associated with teething. The American Academy of Pediatric Dentistry officially discourages the use of any prescription or over-the-counter numbing agents in teething children. These products carry potential toxicity due to the amount that young children ingest. In addition, they are of little benefit as they wash out of the mouth within minutes. For safer alternatives try using chilled teething rings or giving your child a cold washcloth to chew.
The FDA has recently issued a statement warning against the use of prescription numbing agents in teething children. Specifically the use of viscous lidocaine has been shown to potentially cause serious harm (including seizure, brain injury, and heart problems) when used on infants and young children as a treatment for discomfort associated with teething. The American Academy of Pediatric Dentistry officially discourages the use of any prescription or over-the-counter numbing agents in teething children. These products carry potential toxicity due to the amount that young children ingest. In addition, they are of little benefit as they wash out of the mouth within minutes. For safer alternatives try using chilled teething rings or giving your child a cold washcloth to chew.
If you have additional questions, please feel free to contact our office by Online Appointment Request or call 214 618 5200.
